HER2 ADC innovation wave! Kelun, Rongchang, Fosun...what are their competitive strengths?
September 10, 2021
It is reported that at the upcoming ESMO this year, the head-to-head DESTINY-Breast03 research results of DS-8201 and T-DM1 will be unveiled in the form of "Latest Breaking Abstract" (LBA, Late-breaking Abstract). This data is considered to be The most important and sensational research results of this conference. The competition of HER2ADC drugs in my country has become fierce. Following the approval of T-DM1 for marketing, Rongchang Biologics' VC48 for injection was approved for marketing by the NMPA on June 9. It has received at least 2 kinds of drugs for treatment. Patients with locally advanced or metastatic gastric cancer overexpressing HER2 during systemic chemotherapy. In addition to Rongchang Biological, Zhejiang Pharmaceutical, Kelun Pharmaceutical, Jiahe Biological, Fosun Pharmaceutical, etc. are also deeply involved in this. In the future, the competitive landscape of HER2ADC will be explored in this article.
(1) T-DM1: The first ADC drug to be marketed in China
Kadcyla (Enmetrastuzumab, T-DM1) is a HER2ADC drug, which was approved by the FDA in 2013 and has been approved for HER2-positive metastatic breast cancer and HER2-positive early breast with residual lesions after neoadjuvant therapy Adjuvant treatment of cancer. In January 2020, T-DM1 was approved for marketing by NMPA, and its indication is adjuvant for patients with HER2-positive early breast cancer who still have aggressive lesions after receiving neoadjuvant therapy based on taxanes combined with trastuzumab treatment. In the structural design, T-DM1 uses trastuzumab, the cytotoxic drug uses the tubulin inhibitor DM1, and the linker uses MCCLinker. As an early ADC drug, T-DM1 has insufficient structural design. In the choice of payload, DM1 has limited toxicity, which is not as good as MMAE and MMAF, which are also tubulin inhibitors. Looking at the ADC drugs on the market, only T-DM1 is used. DM1 is used as the payload; in addition, due to the use of an uncleavable linker, although the stability is improved, it does not have the bystander effect (BystanderEffect).
From the mechanism of action, T-DM1 can bind to the subregion IV of the HER2 receptor on the cell membrane surface. After being endocytosed and degraded by lysosomes, it releases the cytotoxin DM1 in the cytoplasm, destroys the cell’s tubulin, and causes the cell Cycle arrest and apoptosis. In addition, after T-DM1 is endocytosed through the cell membrane, it enters the endosome, part of T-DM1 binds to FcRn, and is discharged outside the membrane through FcRn-mediated recycling, increasing the half-life of the drug.
Figure: T-DM1 mechanism of action
Data source: Roche
Since its launch, with the broad patient population and unique treatment mechanism of breast cancer, T-DM1 global sales have continued to rise. In 2020, global sales of 1.745 billion Swiss francs, close to 2 billion U.S. dollars, increased by 25% year-on-year. However, in the domestic market, the sales of T-DM1 are hardly optimistic. According to the wind medical library, the sales of T-DM1 in sample hospitals in 2020 is only 16.65 million yuan. On the one hand, the price of T-DM1 is higher, even if you consider the gift of medicine. It is planned that the annual treatment cost is still as high as 260,000 yuan; on the other hand, the indications are narrow and partial, and neoadjuvant chemotherapy in the indications limits the number of patients.
(2) Vidicituzumab: the first domestic ADC drug to be marketed
Vermicutuzumab (RC48) is a HER2ADC drug independently developed by Rongchang Biotech. On June 9, 2021, Vermicutuzumab was approved for marketing by NMPA. The indication is HER2 overexpression that has received at least two systemic chemotherapy. Patients with locally advanced or metastatic gastric cancer. On August 26, the CDE official website announced that the listing application for new indications of vedicitumumab will be included in the priority review for those who have previously received systemic chemotherapy and whose HER2 expression is 2+ or 3+ in immunohistochemical examination. Patients with locally advanced or metastatic urothelial carcinoma (UC). This indicates that vedicitumumab is expected to accelerate the approval of the second indication. From the structural analysis, the mAb of vedicitumumab adopts Disitamab, with an EC50 value of 6.4pM, which has a stronger affinity than trastuzumab and a lower clinical compliance dose; the payload adopts MMAE, which has higher toxicity and can be better. Block tubulin polymerization; using a cleavable linker, the neighboring tumor cells can be killed by the bystander effect.

Figure: The molecular structure of Vidicuzumab
Data source: Rongchang Biological
The indications of T-DM1 for gastric cancer have been broken, and Vidicuzumab provides a new treatment for patients with advanced gastric cancer. According to the Phase Ⅱ clinical study of vedicitumumab for gastric cancer, the ORR of vedicitumumab for third-line treatment of gastric cancer was 24.4%, the median PFS was 4.1 months, and the median OS was 7.9 months. In addition, for patients with urothelial cancer who have undergone second-line and above system chemotherapy, vedicitumumab treatment has shown good efficacy and survival benefits, with an ORR of 50%, an OS of 14.2 months, and mPFS of 5.1 months.
(3) DS-8201: Four surprises with dazzling data
Enhertu (DS-8201) is a masterpiece in the field of HER2ADC, jointly developed by Daiichi Sankyo and AstraZeneca. DS-8201 has created many miracles, and it can be said that it has changed many old concepts in the ADC field on its own. The most worth mentioning is that the DAR of DS-8201 is as high as 8, that is, each antibody molecule is coupled with 8 cytotoxic drugs, which breaks the traditional design concept that DAR should be controlled within 2-4. The combination of high DAR and moderately toxic payload can not only exert better anti-tumor effect, but also reduce off-target toxicity. The choice of DNA topoisomerase inhibitors takes into account both safety and efficacy. In the choice of payload, DS-8201 uses the DNA topoisomerase inhibitor DXd, whose activity is 10 times that of irinotecan. Compared with traditional tubulin inhibitors, DNA topoisomerase inhibitors have more advantages , Including low toxicity, short half-life, and not easy to accumulate in the body; a small number of targets, DNA inhibitors can play a better killing effect when ADC drugs carry the same number of warheads into the cell.
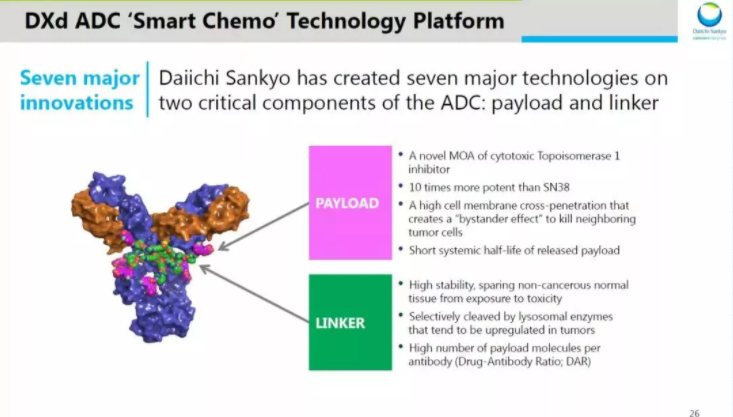
Figure: DS-8201 structure
In 2019, DS-8201 was approved by the FDA as an indication for HER2-positive breast cancer. Experimental data showed that among 184 patients who received the recommended dose, the ORR assessed by IRC reached 60.9%, of which CR reached 6%, PR reached 54.9%, and DoR reached 14.8 months. In terms of safety, DS-8201 has AEs such as decreased neutrophil count, anemia, and interstitial pneumonia. In general, the data of DS-8201 in the treatment of HER2-positive breast cancer is significantly better than T-DM1, but the adverse events of interstitial pneumonia are worthy of attention in clinical use. In 2020, DS-8201 once again won the HER2-positive gastric cancer indication, and the data is extremely eye-catching. The DESTINY-Gastric01 trial showed that DS-8201 third-line treatment for HER2-positive gastric cancer had an ORR of 40.5%, a CR of 7.9%, a PR of 11.3, and a DoR of 11.3 months. With this data, DS-8201 stands out from the strongest. So far there is no marketed drug that can match it. Of course, the indications of interstitial pneumonia are also worthy of vigilance.
(4) ARX788: Site-specific coupling technology of non-natural amino groups
The domestic development and commercialization rights of ARX788 belong to Xinma Bio, a subsidiary of Zhejiang Pharmaceuticals, which is quoted from AMBRX, a leading ADC drug company in the United States. ARX788 is a combination of trastuzumab and AS269. AS269 belongs to the Auristatins toxoid with strong toxicity. On May 18, ARX788 breast cancer indications were included in the breakthrough treatment category. On August 10, the first case of ARX788 gastric cancer phase II/III clinical trial was completed. The core technology of ARX788 is the site-specific coupling technology of unnatural amino acids, which can generate ADC drugs with a DAR of 2 to improve the uniformity of the drugs. Site-specific coupling is a concept relative to random coupling. Since each antibody has several or more amino acid sites, it is difficult to control the connection of toxin drugs. The improvement of DAR weakens the uniformity of drugs. The design of site-specific coupling Usually two special amino acid sites are introduced to more effectively control the connection of antibodies and toxin drugs. The Ambrx unnatural amino acid technical route is divided into three steps: the first step is to synthesize natural amino acids in vitro that meet the requirements of the preparation of medicines; the second step is under the action of synthetase, the natural amino acids are connected with special tRNAs and transported to the ribosome, and the third step tRNA recognizes special codons and precisely inserts natural amino acids into specific parts of the polypeptide chain.
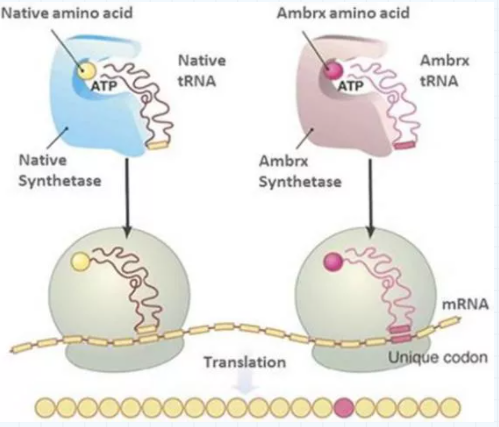
Figure: Schematic diagram of unnatural amino acid site-directed coupling technology
(5) A166: Lysine site-specific coupling technology
A166, developed by Kelun Pharmaceutical Holdings subsidiary Kelun Botai, is the third generation of antibody conjugate drugs targeting HER2. The new toxin molecule (Duo-5, tubulin inhibitor) can be site-specifically coupled by protease cleavable Linker. Linked to HER2 antibody (trastuzumab). At ASCO in 2021, Kelun Pharmaceutical announced the results of the Phase I clinical study of A166. At effective doses of 4.8 and 6.0 mg/kg, the ORRs of 36 patients with evaluable HER2-positive breast cancer were 59.1% (13/22). ) And 71.4 (10/14); 4 patients with evaluable HER2 low expression breast cancer resulted in 1 PR, 2 SD and 1 PD. The median PFS was not reached, and one patient in the 4.8 mg/kg dose group had received A166 treatment for more than 19 months. In terms of safety, A166 is generally well controllable, but there are adverse ocular events. For ocular toxicity, it can be recovered after symptomatic treatment.
(6) Summary
Affected by the global innovation wave and the update and iteration of ADC drugs, HER2ADC has become one of the most competitive areas in the domestic ADC field. Rongchang Biological, Zhejiang Pharmaceutical, Kelun Pharmaceutical, Jiahe Biological, Fosun Pharmaceutical, etc. are all located here. In addition to HER2ADC, BeiGene's ZW49 is a HER2×HER2ADC that can simultaneously bind to two non-overlapping epitopes of HER2, which is expected to improve the targeting of drugs. However, the research and development of HER2ADC drugs has not been smooth sailing. Biotech’s BAT8001 has already broken down. In the future, companies related to HER2ADC will need to race against time on the one hand and accelerate R&D to market first; on the other hand, they will adopt a differentiated path and multi-indication layout. There have been many breakthroughs in indications for breast cancer, stomach cancer, and urothelial cancer.






