The gene therapy market broke out! The market size will exceed 30.54 billion U.S. dollars in 2025
June 15, 2021
Since 2015, the global gene therapy industry has accelerated its development and continued to make breakthroughs. Especially after 2017, with the launch of the adeno-associated virus drug Luxturna and two CAR-T drugs Kymriah and Yescarta, the gene therapy industry has developed rapidly and has become one of the most promising global frontier medical fields. Gene therapy is to treat diseases caused by gene defects or abnormal expression by introducing normal genes or modifying or manipulating gene expression. The development of gene therapy began on September 14, 1990, when a clinical trial began to treat the first patients with adenosine deaminase deficiency and severe combined immunodeficiency (ADA-SCID).
History of gene therapy
Picture source: Heyuan Biological Prospectus
Explosive growth of global gene therapy
14 models are on the market; more than 1,300 items are under clinical research
With the development of gene therapy, the safety and effectiveness of the vectors used in gene therapy products have gradually improved. A number of gene therapy products have been approved for marketing. According to incomplete statistics from Yaozhi.com, up to now, the genes that have been approved for sale worldwide have been approved for marketing. There are 14 types of treatment drugs. In 2020, the combined sales of Zolgensma and Kymriah, the world's leading gene therapy drugs, for Novartis, have reached US$1.4 billion.
Gene therapy drugs approved for sale worldwide
Data source: Yaozhi data, FDA, EMA, etc. (Note: The above data is incomplete statistics. If there are omissions, please add them)
Among them, two domestic gene therapy products have been launched in China, namely recombinant human p53 adenovirus injection (trade name: Jinyousheng) and recombinant human adenovirus type 5 injection (trade name: Ankerui); it is worth mentioning However, Jinshengsheng was developed by Shenzhen Sibainuo Company and was approved by CFDA in 2003. It is the world's first anti-tumor gene therapy product. In addition, at present, the fastest progress in China is Fosun Kate’s CAR-T product-Yiqi Lilunsai injection, which is a target that Fosun Kate introduced Yescarta technology from KitePharma in the United States and was authorized to conduct localized production in China. For CD19 autologous CAR-T cell products, its marketing application will enter the administrative approval stage on January 12, 2021. At the same time, driven by technology, capital and policies, the global gene therapy industry is rapidly heating up, and a large number of gene therapy drug research and development have entered the clinical stage, and have shown explosive growth since 2015. According to ASGCT data, as of the end of 2020, there have been more than 1,300 clinical trials of gene therapy under research worldwide; about 25% of them are in clinical phase II-III, and 6% are in clinical phase III.
Picture source: Heyuan Biological Prospectus
The global gene therapy market will exceed 30 billion U.S. dollars
China has become the second largest research country after the United States
According to Frost & Sullivan's analysis, it is estimated that by 2025, the global gene therapy market will reach nearly 30.54 billion U.S. dollars, and China will reach 17.89 billion U.S. dollars.
According to ASGCT data, as of the end of 2020, the United States is the country with the largest number of gene therapy clinical trials, with a total of more than 650; followed by China, with a total of more than 300. China has become one of the main markets for gene therapy drug research and development, and the number of clinical studies is second only to the United States, ranking second in the world.
Picture source: Heyuan Biological Prospectus
In addition, according to incomplete statistics from Yaozhi data, as of now, more than 20 gene therapies in China have entered the clinical stage, and the indications are mainly concentrated on rare diseases and solid tumor diseases such as melanoma. Domestic gene therapy drug research and development companies include Boya Gene, Fosun Kate, Legend Bio, WuXi Junuo, etc., most of which focus on CAR-T, TCR-T and other immune cell therapies.
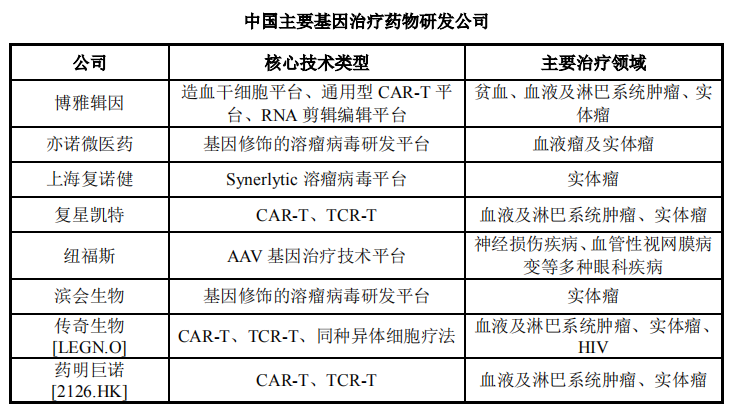
Picture source: Heyuan Biological Prospectus
Fosun Kate and WuXi Junuo’s two CAR-T drugs, Yiqirensai and Ruijirensai, have been submitted for marketing and have received priority review and breakthrough therapy designation respectively. Yiqirensai has entered the market The approval status is expected to become the first approved CAR-T therapy in China. Although there are certain differences between the domestically approved gene therapies and the international ones, the domestic gene therapy is currently on the rise, and clinical research on related drugs is in full swing. It is expected that there will be continuous registration and development of new gene therapy drugs in the future. The clinical research phase will continue to advance, and it is foreseeable that gene therapy will accelerate its decline in the domestic market in the near future.
Data and information sources: public information and data such as Yaozhi.com, Yaozhi Data, ASGCT data, and Metabiological Prospectus








