15 blockbuster drugs expire patents! BMS accounted for 3 models, Merck & Co., Ltd. 2 models, AbbVie, Novartis, Pfizer...
July 16, 2021
In 10 years, the situation is changing, and the pharmaceutical field will also undergo significant changes. In particular, a batch of super "blockbusters" will fall off the cliff of patents. This allows pharmaceutical giants to sit back when investors applaud their next blockbuster drug. In a more awkward position. Recently, Fierce Pharma has released a list of blockbuster patented drugs whose patents will expire within 10 years. Among them, 9 drugs will be the best-selling drugs in the world in 2020, including Humira, Creta, Refumei, and Electro Global The top 4 best-selling blockbuster drugs with annual sales of tens of billions of dollars are included. From a corporate perspective, Bristol-Myers Squibb will be the drugmaker with the most drugs that may lose patent exclusivity in the past ten years. The blockbuster products Ruifumei, Elto and Odivo will all face a patent cliff; secondly, Merck has two In addition, the global drug repair Merlot will also face patent expiration in 2023, with a market of nearly 20 billion U.S. dollars. With only one and a half years left, whether the drug patent expires Preparations for post-generic drugs to participate in the competition?
List of TOP15 blockbuster drugs with patents expiring within ten years
01,Humira: AbbVie, sales in 2020: $19.8 billion (US$16.1 billion), key patent expiration date: 2023, Humira is the world’s first fully human monoclonal Antibodies were approved by the U.S. FDA in 2002. Since 2012, Humira has won the world's top sales of prescription drugs for seven consecutive years. In 2020, it generated US$19.8 billion in global revenue. Last year's sales in the United States were 16.1 billion US dollars; generated about 43% of total revenue for AbbVie.
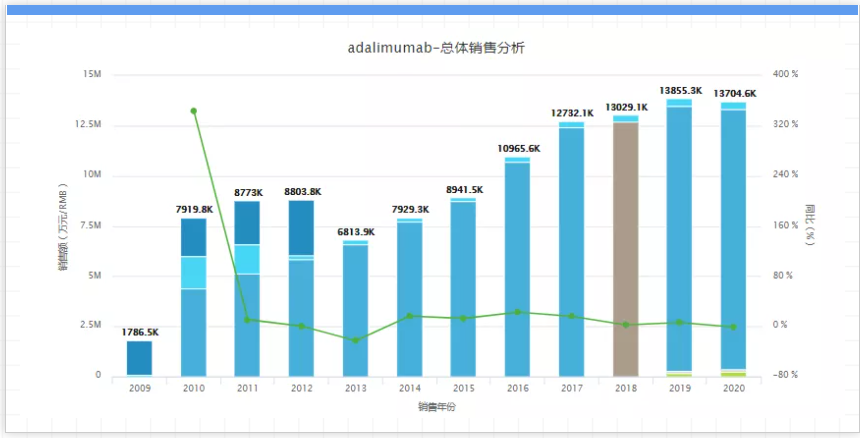
As Humile brought huge income to AbbVie, AbbVie worked very hard to defend the patent right of Adalimumab. But since AbbVie signed the first Humira biosimilar agreement with Amgen in September 2017, its patent war has been full of gun smoke. A total of at least eight Humira biosimilar agreements have been signed, including Boehringer Ingelheim, Pfizer, Samsung Biologics, Mylan, Sandoz, etc. finally compromised and shared the market. Adalimumab biosimilars could enter the US market as early as 2023. With only one and a half years left before the patent expiration date, a large number of biosimilar drugs may be listed in the United States, how AbbVie can solve the predicament of Humira's patent cliff, and whether it can continue to sit on the throne of "drug king", wait and see! 02 Keytruda: Merck, 2020 sales: 14.38 billion US dollars, key patent expiration time: 2028, Keytruda is a PD-1 inhibitor for the treatment of non-small cell lung cancer (NSCLC ), small cell lung cancer (SCLC), head and neck cancer, hepatocellular carcinoma (HCC), kidney cancer and more than 10 cancer indications. In 2020, sales increased by 30% year-on-year, and total sales reached 14.4 billion U.S. dollars.
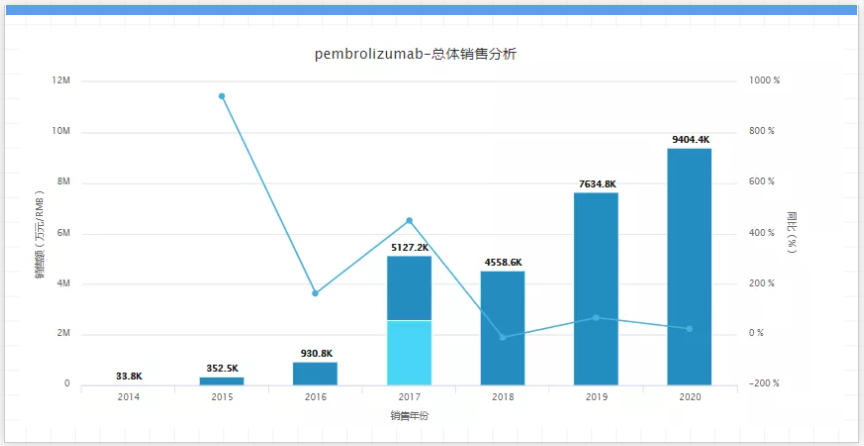
According to Merck, the development of Keytruda is still at an early stage! As of the end of March this year, there have been more than 1,450 clinical trials tested in more than 30 cancer types to test the efficacy of Keytruda in the treatment of cancer patients in different environments, including more than 1,050 trials for combination therapy clinical trials. In addition, according to the Evaluate Pharma analysis report, Keytruda is expected to replace AbbVie's Humira in 2023 as the world's best-selling drug, and is expected to reach US$24.32 billion in global sales in 2026. However, the more successful Keytruda becomes today, the more Merck needs to be prepared before the drug falls off the patent cliff in 2028. Biosimilars are already in the pipeline. A Canadian company called PlantForm Corporation recently announced a collaboration with Bio-Manguinhos/Fiocruz of the Brazilian Ministry of Health to develop a biosimilar of Keytruda for the Brazilian market. In addition, Sydney-based neuron Pharmaceuticals and Serum Institute of India have collaborated on 10 biosimilar projects, one of which is based on Keytruda. 03. Revlimid, company: Bristol-Myers Squibb, sales in 2020: $12.1 billion, key patent expiration: 2025-2026. Revlimid is an immunomodulatory agent used to fight tumors and control tumor cell proliferation. It is used to treat multiple myeloma, myelodysplastic syndromes and other diseases. (Acquisition) A new generation of anti-tumor drugs developed by Pharmaceuticals was approved by the U.S. Food and Drug Administration (FDA) for marketing in 2006.
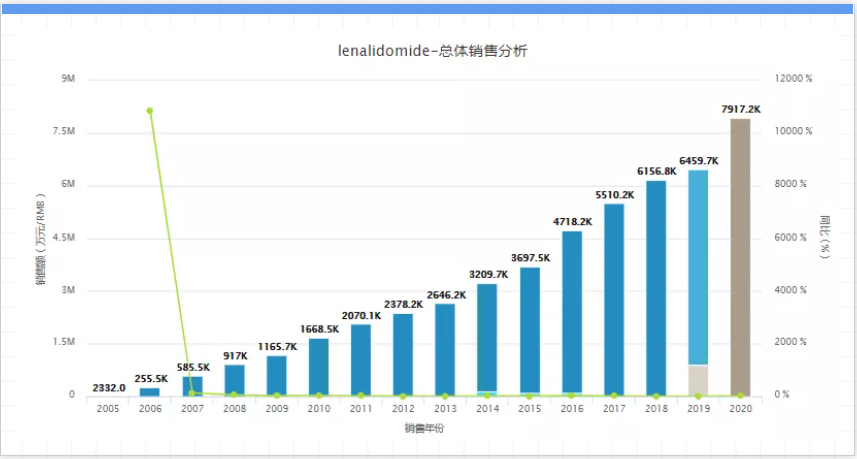 In 2020, Revlimid’s global sales will reach US$12.15 billion. According to Moody’s analysis and prediction, although Revlimid is currently the third best-selling drug in the world, second only to Humira and Keytruda, it will continue to grow with generic competitors from 2025 to 2026. Join the competition between, this kind of limelight is quickly fading. The first imitator comes from India’s Natco Pharma, which may be launched as early as March 2022. Although the number is limited, there will be more generic drugs on the market sometime soon thereafter. 04. Eliquis, company: Bristol-Myers Squibb & Pfizer, sales in 2020: US$9.2 billion, key patent expiration date: 2027 to 2029, Eliquis is jointly developed by Pfizer and Bristol-Myers Squibb An anticoagulant was first approved for marketing in Europe in 2011 and then launched in the United States in 2012. It is used to reduce the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation. It can also be used to prevent the formation of deep vein thrombosis. Since its approval for listing, Eliquis has become a star of Pfizer and BMS, with sales of 9.17 billion U.S. dollars in 2020, an increase of 16% over 2019, and a place in the top five best-selling drugs in the world in 2020.
In 2020, Revlimid’s global sales will reach US$12.15 billion. According to Moody’s analysis and prediction, although Revlimid is currently the third best-selling drug in the world, second only to Humira and Keytruda, it will continue to grow with generic competitors from 2025 to 2026. Join the competition between, this kind of limelight is quickly fading. The first imitator comes from India’s Natco Pharma, which may be launched as early as March 2022. Although the number is limited, there will be more generic drugs on the market sometime soon thereafter. 04. Eliquis, company: Bristol-Myers Squibb & Pfizer, sales in 2020: US$9.2 billion, key patent expiration date: 2027 to 2029, Eliquis is jointly developed by Pfizer and Bristol-Myers Squibb An anticoagulant was first approved for marketing in Europe in 2011 and then launched in the United States in 2012. It is used to reduce the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation. It can also be used to prevent the formation of deep vein thrombosis. Since its approval for listing, Eliquis has become a star of Pfizer and BMS, with sales of 9.17 billion U.S. dollars in 2020, an increase of 16% over 2019, and a place in the top five best-selling drugs in the world in 2020.
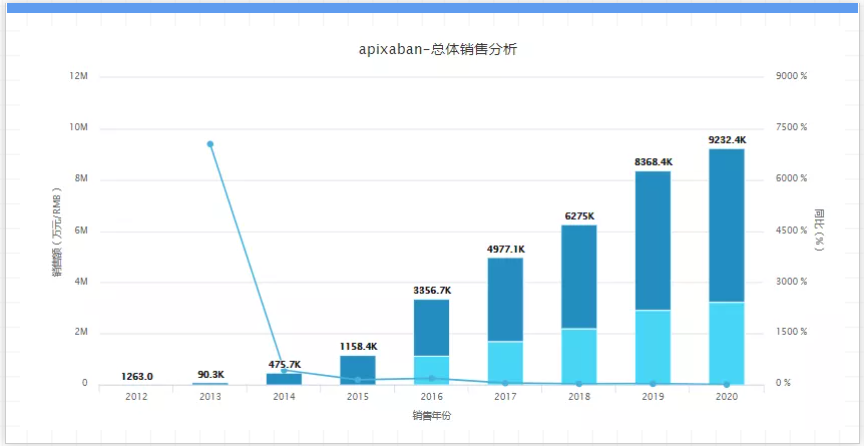
But for many years, Pfizer and Bristol-Myers Squibb have been working hard to fight against competitors of generic drugs. In 2017, 25 companies applied for FDA approval of their generic drugs. In August of the same year, the U.S. Patent and Trademark Office granted Eliquis a key substance component patent, extending its patent from February 2023 to November 2026. In addition, the anticoagulant drug has another formulation patent, which is valid until 2031. At present, it is not clear when Eliquis generic drugs will begin to penetrate, but BMS said that it expects generics to enter after 2026 and before 2031. 05. Eylea, the company: Regeneron, Bayer, 2020 sales: 8.36 billion US dollars, key patent expiration time: 2025 to 2026, Eylea is the world's first fully humanized fusion Protein, a therapeutic drug for the treatment of diabetes-related retinopathy. Regeneron sells the drug in the United States, and the German Bayer Group sells the drug overseas. In 2020, Ariya's global sales reached 8.36 billion U.S. dollars, an increase of 7% over 2019.
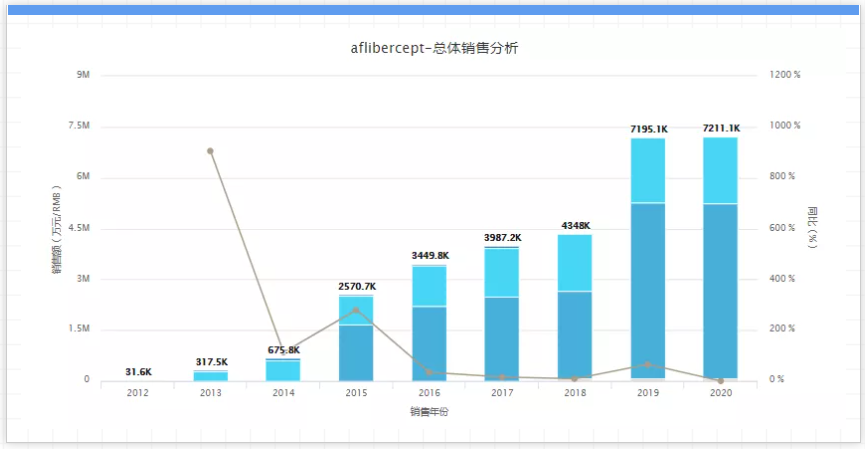
It is reported that South Korea's Samsung Bioepis began its late-stage research on its Eylea biosimilar SB15 at the end of June last year, and the trial is expected to end in April next year; South Korean companies such as Alteogen, Celltrion and Samchundang Pharm are also actively researching Eylea similar drugs. In addition, Sandoz said in May this year that it will soon start the registration of the Phase 3 clinical trial of Eylea similar drugs; Amgen also expects to complete the Phase 3 clinical trial of Eylea similar drugs in April 2022. It is worth noting that there is also a potential threat to Roche Lucentis (Ranibizumab) biosimilar drug, Samsung Bioepis/Bojian’s SB11, which has been approved by the FDA for biological product approval. If it is successfully approved for marketing in the second half of 2021, It may grab a large portion of Eylea's market share at a low price. 06. Stelara, company: Johnson & Johnson, sales in 2020: 7.7 billion US dollars, key patent expiration time: 2025 to 2026, Stelara is a monoclonal antibody drug, a major Johnson & Johnson Pound anti-inflammatory drugs, used to treat plaque psoriasis, psoriatic arthritis and Crohn’s disease, global sales in 2020 reached 7.7 billion U.S. dollars, an increase of 21% over 2019. But it eventually had to face competition from generic drugs after the patent expired.
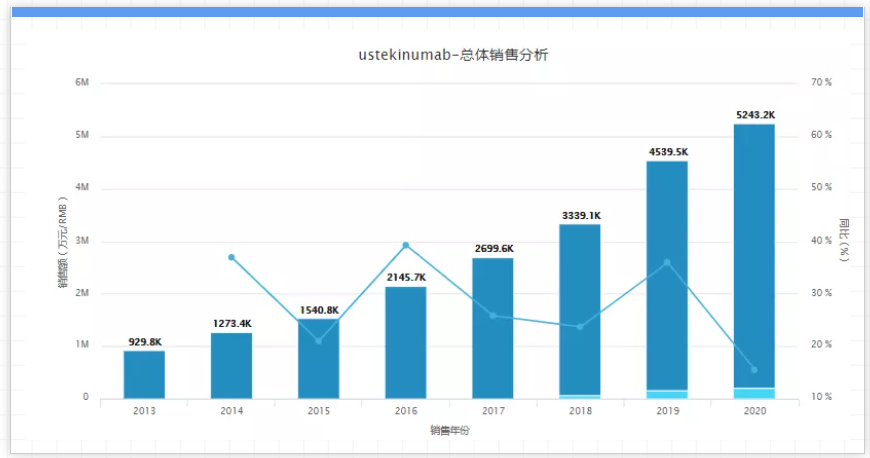
It is reported that South Korea’s Celltrion is the company with the fastest research progress among its competitors. Its candidate products have entered clinical phase III. The research is expected to end in the second half of 2022. If successful, it will be launched in 2023 or 2024. In addition, it is reported that several other biosimilar manufacturers, including Bio-Thera Solutions, Epirus biopharmacpharmaceuticals and NeuClone, are also conducting early trials of similar drugs to the drug. 07. Opdivo, company: Bristol-Myers Squibb, 2020 sales: 6.99 billion US dollars, key patent expiration time: 2028. Opdivo, the world's first approved PD-1 inhibitor, has been ranked among the world's best-selling drugs since it was approved for marketing, and has become a star drug that Bristol-Myers Squibb is doing its part. It has become the most direct competitor with Keytruda. However, due to the hindrance of its research and development in non-small cell lung cancer, Opdivo's sales in recent years have been surpassed by Keytruda. In 2020, Opdivo was only 7 billion US dollars, down 3% from the previous year.
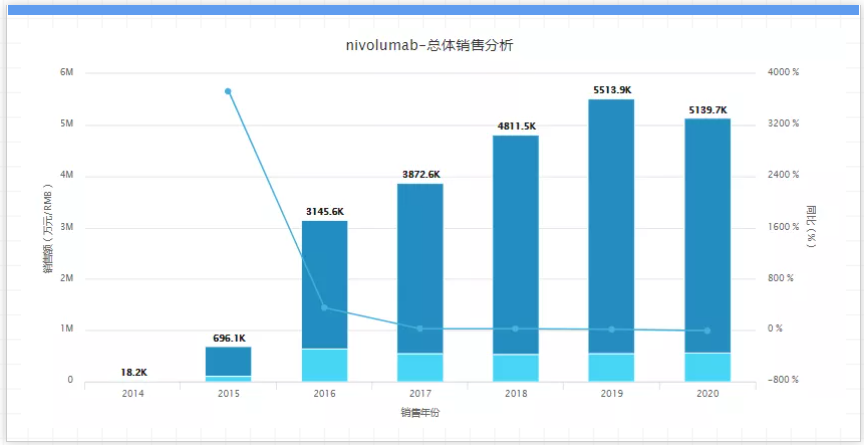
In recent years, in order to consolidate Opdivo's position, BMS has been expanding the indications of Opdivo and expanding combination therapy with other drugs. However, it has encountered many failures in expanding its indications. According to the securities documents recently submitted by BMS, Opdivo will lose its patent protection in the United States in 2028. In addition, Sydney NeuClone, Sweden Xbrane, and China's Luye Pharma are already developing Biosimilar drugs. With competitors and patent cliffs approaching, whether Opdivo can stabilize its market prospects in the next time, it still needs to make major breakthroughs in clinical research instead of repeated setbacks. 08, Dolutegravir, company: GlaxoSmithKline, sales in 2020: 6 billion US dollars, key patent expiration time: 2027-2029. Dolutegravir is a new generation of HIV treatment drug developed by VIIV HLTHCARE. It was approved for marketing by the US FDA on August 12, 2013. It is one of GSK's best-selling HIV brand drugs, and its sales in 2020 accounted for a quarter of GSK's total, reaching US$6 billion. However, with the expiration of its molecular patent in 2027 and its crystal form patent in 2029, Dolutegravir had to face the challenge of generic drugs. It is reported that in 2014, Medicines Patent Pool (MPP, Medicines Patent Pool) signed a related agreement with VIIV, enabling generic drug manufacturers to produce low-cost versions of DTG for countries with the highest HIV burden. As of the end of 2020, DTG for pediatrics in 121 countries and DTG for adults in 95 countries have obtained MPP-ViiV imitation agreement authorization (namely patent voluntary license agreement). In addition, in the HIV field, GSK is facing competition from the two giants Gilead and Merck. In March of this year, the two companies announced plans to combine their two antiviral drugs, lenacapavir and islatravir, to develop a weekly oral therapy and Injection therapy every three months; and encouraging data have been obtained. In order to cope with the loss of key patent expiration and fill the gap in the market, GSK focuses on more convenient long-acting injections instead of daily pills, but the final result is whether it can stabilize its market position in the HIV field and wait for its effects. 09. Ibrance, company: Pfizer, sales in 2020: $5.39 billion, key patent expiration date: 2027. Ibrance is the world's first CDK4/6 inhibitor approved as a cancer therapy for the combined treatment of advanced or metastatic HR+/HER2- breast cancer in postmenopausal women. It has always been Pfizer's blockbuster drug, with sales in 2020 reaching 5.39 billion U.S. dollars, a year-on-year increase of 9%.
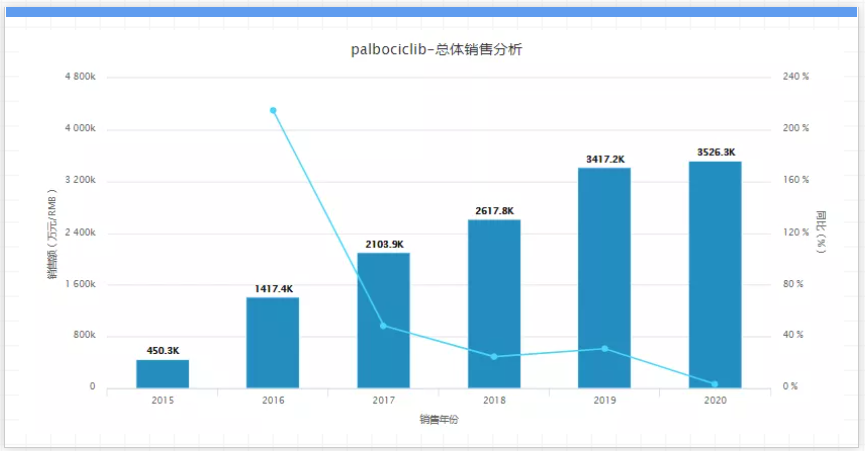
At present, Ibrance is facing two major competitors, Novartis’s Kisqali and Eli Lilly’s Verzenio; in addition, two clinical failures of Ibrance as an adjuvant therapy have been announced one after another in 2020; the fall of the former gives the latter a chance to surpass; Eli Lilly’s Verzenio may be the first CDK4/6 inhibitor to prevent the recurrence of HR+/HER2-early breast cancer. According to data from Pfizer's annual report, in the United States, Ibrance has occupied nearly 90% of the CDK inhibitor market share and 80% of the first-line CDK inhibitor market. The annual sales of Novartis and Eli Lilly’s CDK4/6 inhibitors have doubled in the past two years and are eroding the Ibrance market. Ibrance will lose its market exclusivity in the United States in 2027. Some generic drug companies will also Already about to move. 10. Januvia & Janumet, company: Merck, sales in 2020: $5.3 billion, key patent expiration time: 2022 to 2023. Januvia & Janumet are Merck
The combined preparations of eastern biguanides and DPP-4 inhibitors have global revenues of 5.3 billion to 6.1 billion U.S. dollars. However, Januvia+Janumet's market sales have stagnated since 2012, especially in 2019, its sales have dropped significantly by 7%, and again in 2020 by 4%. Over the years, Merck has spared no effort to protect its Januvia and Janumet (sitagliptin and metformin) patents. However, the generic version of sitagliptin by many companies including Sun Pharma and India’s Zydus Cadila has been approved by the FDA and will be released in Merck’s patent will be launched when its patent expires. In addition, Eli Lilly’s Trulicity (dulaglutide) and Novo Nordisk’s Ozempic (smeglutide) are also competitors that cannot be ignored. As the patent expires, The old tree of small molecule DPP-4 inhibitors may not escape the cycle of drug life cycle. 11, Trulicity, company: Eli Lilly, sales in 2020: $5.1 billion, key patent expiration: 2027 From 2029 to 2029, Trulicity is a GLP-1 receptor agonist that Eli Lilly has high hopes for. It entered the market in September 2014 and exceeded US$5 billion in sales for the first time, becoming the world's 15th best-selling drug; reaching 50.7 Billion U.S. dollars, and overall sales increased by 4%.
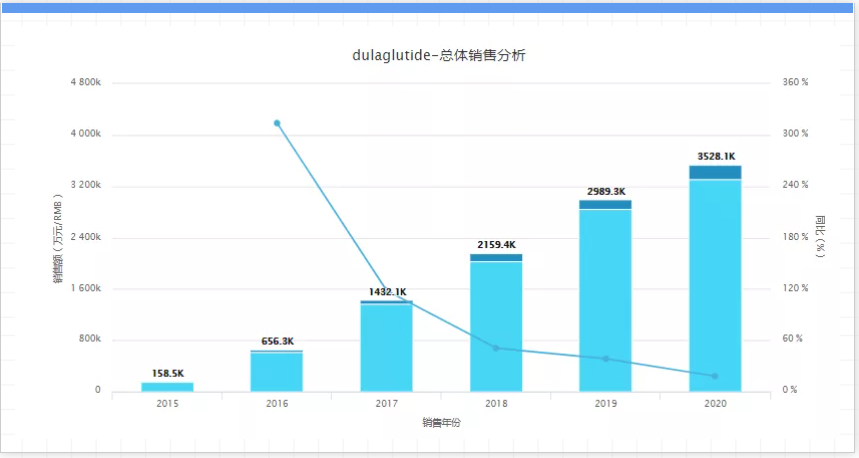 Although Eli Lilly’s Trulicity is still advancing all the way, with the passage of time, it has to face the pressure of patent expiration, especially Novo Nordisk’s Ozempic (Smegaglutide). However, according to the published data, in a head-to-head clinical study, Trulicity defeated AstraZeneca’s Byetta and Merck’s Januvia/metformin combination therapy, and also proved that its efficacy is better than Novo Nordisk’s blockbuster drugs. Victoza. And is working hard to develop it hopes to become the next diabetes heavyweight drug, that is, the GIP/GLP-1 dual agonist tirzepatide. 12. Prolia/Xgeva, company: Amgen, sales in 2020: 4.6 billion US dollars, key patent expiration time: 2025-2026. Denosumab is a human immunoglobulin G2 (IgG2) monoclonal antibody. It is the first biologic for osteoporosis. It was developed by Amgen and is currently approved for multiple indications worldwide. In 2020, sales of 4.6 billion U.S. dollars will be achieved.
Although Eli Lilly’s Trulicity is still advancing all the way, with the passage of time, it has to face the pressure of patent expiration, especially Novo Nordisk’s Ozempic (Smegaglutide). However, according to the published data, in a head-to-head clinical study, Trulicity defeated AstraZeneca’s Byetta and Merck’s Januvia/metformin combination therapy, and also proved that its efficacy is better than Novo Nordisk’s blockbuster drugs. Victoza. And is working hard to develop it hopes to become the next diabetes heavyweight drug, that is, the GIP/GLP-1 dual agonist tirzepatide. 12. Prolia/Xgeva, company: Amgen, sales in 2020: 4.6 billion US dollars, key patent expiration time: 2025-2026. Denosumab is a human immunoglobulin G2 (IgG2) monoclonal antibody. It is the first biologic for osteoporosis. It was developed by Amgen and is currently approved for multiple indications worldwide. In 2020, sales of 4.6 billion U.S. dollars will be achieved.
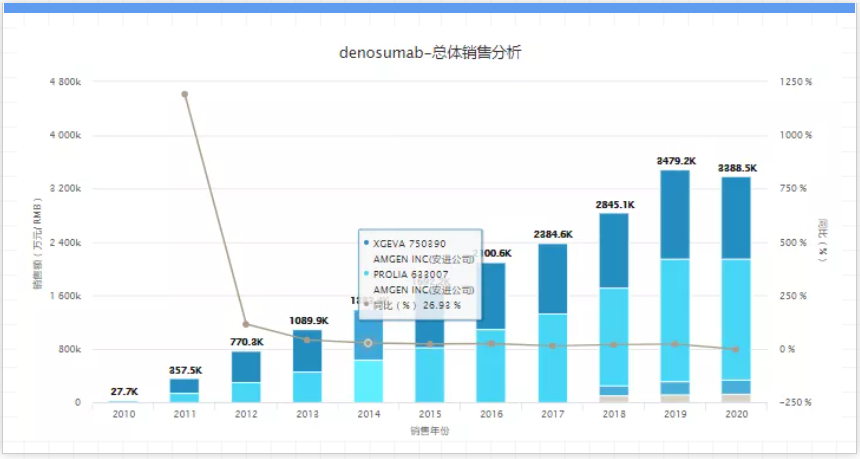 But according to its annual documents, Amgen listed the expiration date of the denosumab patent between 2021 and 2025. Sandoz, Samsung biopeis, and Taiwan's JHL Biotech have begun to imitate denosumab; and Sandoz similar drugs are undergoing phase 3 trials, which are expected to end in July 2022. However, in order to alleviate the pain of declining sales due to patent expiration, Amgen has been making arrangements. Evenity, a new-generation osteoporosis drug launched in 2019, achieved a growth of 85% last year, reaching US$350 million. 13. Cosentyx, company: Novartis, sales in 2020: USD 4 billion, key patent expiration time: 2025 to 2026, Cosentyx is the world's first interleukin-17A (IL-17A) inhibitor, It is the world's first IL-17A monoclonal antibody approved for listing. In the following year, Cosentyx became a blockbuster with annual sales of 1.128 billion U.S. dollars. In 2020, its sales reached 3.995 billion U.S. dollars. Cosentyx is already Novartis's sales ranking. The first product.
But according to its annual documents, Amgen listed the expiration date of the denosumab patent between 2021 and 2025. Sandoz, Samsung biopeis, and Taiwan's JHL Biotech have begun to imitate denosumab; and Sandoz similar drugs are undergoing phase 3 trials, which are expected to end in July 2022. However, in order to alleviate the pain of declining sales due to patent expiration, Amgen has been making arrangements. Evenity, a new-generation osteoporosis drug launched in 2019, achieved a growth of 85% last year, reaching US$350 million. 13. Cosentyx, company: Novartis, sales in 2020: USD 4 billion, key patent expiration time: 2025 to 2026, Cosentyx is the world's first interleukin-17A (IL-17A) inhibitor, It is the world's first IL-17A monoclonal antibody approved for listing. In the following year, Cosentyx became a blockbuster with annual sales of 1.128 billion U.S. dollars. In 2020, its sales reached 3.995 billion U.S. dollars. Cosentyx is already Novartis's sales ranking. The first product.
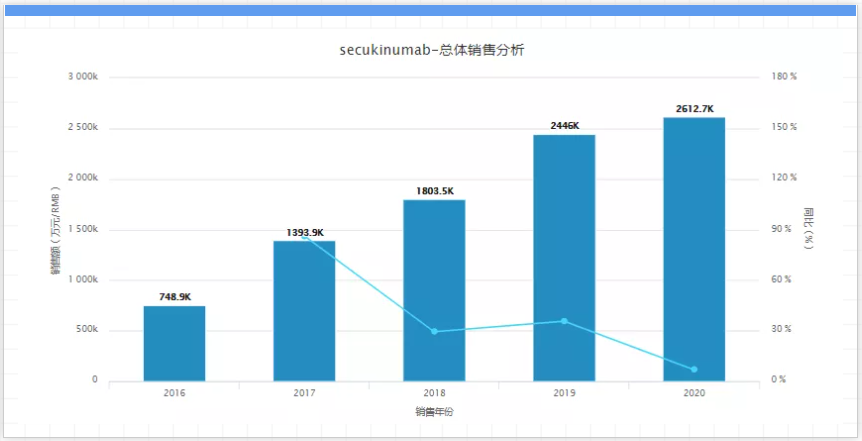 It is reported that up to now, four drugs with the same target, including Cosentyx, have been listed (the other three are: Taltz, Siliq, and Efleira). In addition, on September 22, 2020, Utimer's IL-17A/IL-17F dual neutralizing monoclonal antibody Bimekizumab has been accepted by the FDA and EMA for the treatment of moderate to severe plaque psoriasis. As the patent period approaches, competition may become more intense. 14. Entyvio, company: Takeda, sales in 2020: USD 4 billion, key patent expiration time: 2025 to 2026
. Entyvio is an intestinal selective humanized monoclonal antibody that was approved for marketing in the United States and the European Union in May 2014 for the treatment of moderate to severe patients with insufficient response to conventional treatment or tumor necrosis factor alpha (TNFα) antagonists Adult patients with active ulcerative colitis (UC) and Crohn's disease (CD). It is also worth mentioning that in 2019, the results of the head-to-head trial between Entyvio and Humira showed excellent efficacy and safety. The transformative trial made Entyvio the leading drug in the UC field, and sales increased from 2019 to 2020. 29%, reaching US$4 billion.
It is reported that up to now, four drugs with the same target, including Cosentyx, have been listed (the other three are: Taltz, Siliq, and Efleira). In addition, on September 22, 2020, Utimer's IL-17A/IL-17F dual neutralizing monoclonal antibody Bimekizumab has been accepted by the FDA and EMA for the treatment of moderate to severe plaque psoriasis. As the patent period approaches, competition may become more intense. 14. Entyvio, company: Takeda, sales in 2020: USD 4 billion, key patent expiration time: 2025 to 2026
. Entyvio is an intestinal selective humanized monoclonal antibody that was approved for marketing in the United States and the European Union in May 2014 for the treatment of moderate to severe patients with insufficient response to conventional treatment or tumor necrosis factor alpha (TNFα) antagonists Adult patients with active ulcerative colitis (UC) and Crohn's disease (CD). It is also worth mentioning that in 2019, the results of the head-to-head trial between Entyvio and Humira showed excellent efficacy and safety. The transformative trial made Entyvio the leading drug in the UC field, and sales increased from 2019 to 2020. 29%, reaching US$4 billion.
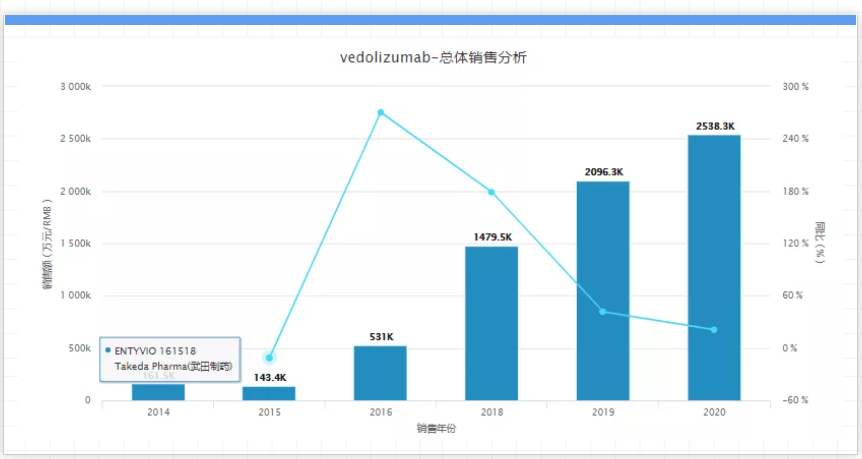
However, the drug will face the adjustment of patent expiration by 2025. In the last few years, how should Takeda Pharmaceutical grasp the period of market exclusivity? 15. Victoza, company: Novo Nordisk, sales in 2020: 3 billion US dollars, key patent expiration time: 2022 to 2023. Victoza is Novo Nordisk's second marketed GLP-1RA hypoglycemic drug in the world. It was approved by the FDA and EMA in June 2009 and January 2010, although it was 4 to 5 years later than exenatide. However, with the support of Novo Nordisk's vigorous promotion and a number of post-marketing clinical trials, liraglutide quickly occupied the GLP-1 market after its listing.
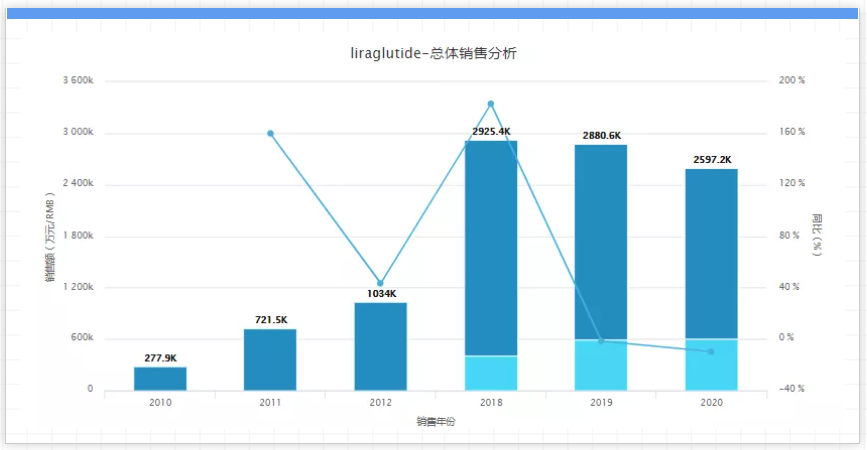
It is worth mentioning that in 2018 and even the previous 7 years, Novo Nordisk’s liraglutide has always occupied the top sales list of GLP-1 receptor agonists. In 2019, it finally gave way to Eli Lilly’s Dura Glycopeptide. In addition, its 2020 annual report stated that Victoza's active ingredient patents will expire in Japan next year, the United States and Germany in 2023, and have expired in China. Perhaps with the advent of the patent cliff, Victoza will have a certain decline in sales, but Novo Nordisk may not immediately face generic competition. It has been intensively working in the GLP-1 field, and Ozempic, Rybelsus and Wegovy have shown good market prospects.
See literature and data:
1. Yaozhi data
2. The top 15 blockbuster patent expirations coming this decade
















