60 billion market size! The fifth batch of centralized procurement of large varieties and leading companies gathered; Pfizer, AstraZeneca, Hengrui, Kelun, Chia Tai Tianqing...
May 18, 2021
Recently, Shanghai Sunshine Pharmaceutical Purchasing Network issued the "Notice on the Implementation of the Fifth Batch of National-Organized Drug Centralized Procurement of Drug Information Collection", and the fifth batch of centralized procurement officially started the application of relevant information. According to the notice, the fifth batch of centralized procurement has a total of 60 kinds and 202 specifications within the scope of declaration, including 30 blockbuster injections. According to Yaozhi data, up to now the fifth batch of centralized procurement involves more than 170 companies, including the original research and reference preparation companies; in terms of the number of varieties, the number of injections, and the number of companies involved, it is the largest national procurement so far. The largest number of times. According to Fengyun Yaotan's forecast, the fifth batch of centralized procurement market will reach close to 60 billion.
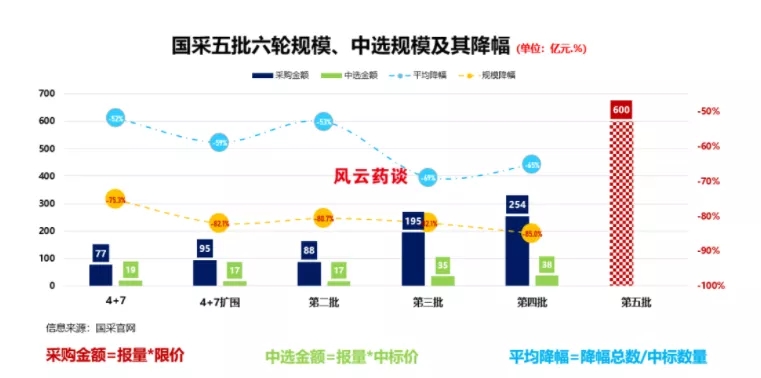
Image source: Fengyun Yaotan
12 varieties of fierce competition, the price of rivaroxaban 2 billion best-selling drug will avalanche?
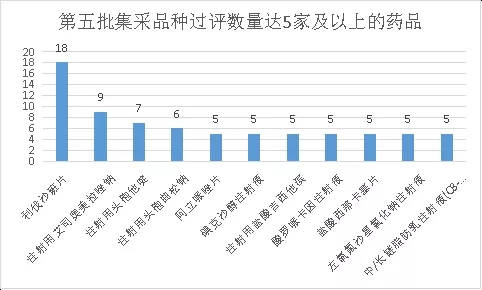
From the perspective of drug varieties, the most competitive product is rivaroxaban tablets. A total of 18 companies have reviewed it (involving 3 specifications). Adding the original research company means that 19 companies meet the application requirements.
Rivaroxaban is a highly effective FXa inhibitor jointly developed by Bayer and Johnson & Johnson. It was first approved for listing in China in 2009, entered the National Medical Insurance List in the same year, and entered the National Essential Drug List in 2018. It is currently a group of people with safe medication experience. The broadest new type of oral anticoagulant. According to previous financial reports, Bayer’s sales of rivaroxaban in markets outside the United States were 4.515 billion yuan; Johnson & Johnson’s domestic market in the United States made a total of 2.345 billion U.S. dollars; therefore, in 2020, the global sales of rivaroxaban will reach 7.5 billion U.S. dollars, which is the top ten in the world. One of the best-selling drugs. According to public data, the Chinese hospital terminal market for rivaroxaban exceeds 2 billion yuan, and it is worth noting that with the expiration of the rivaroxaban compound patent in 2020, domestic generic drugs have been approved for listing. Up to now, there are 18 The company is approved for listing and is deemed to have passed the consistency evaluation. Chia Tai Tianqing is the first imitation company. Now the product has been included in the fifth batch of collective procurement list. The price war is about to start. Will the price of rivaroxaban be an avalanche? Will the first generic drug get dividends as promised?
Rivaroxaban Pass/Deemed Pass Consistency Evaluation Details Table
30 injections, 8 varieties, more than 5 over-rated companies
In terms of the drug formulations of this centralized procurement, injections are undoubtedly the most interesting object. This centralized procurement has a total of 30 injections, accounting for 50% of the total, which is the largest collection of injections in the past. There is no shortage of iodixanol injections, cefuroxime injections, ceftriaxone injections, ceftazidime injections, cefazolin injections, esomeprazole (esomeprazole) injections and other large varieties with a market share of over 3 billion. . According to the data of Yaozhi, there are more than 5 companies with 8 varieties of injections that have been reviewed, namely esomeprazole sodium for injection, iodixanol injection, gemcitabine hydrochloride for injection, and ropivacaine hydrochloride injection. Liquid, ceftriaxone sodium for injection, ceftazidime for injection, levofloxacin sodium chloride injection, medium/long chain fat emulsion injection (C8-24); among them, esomeprazole sodium for injection is the most competitive injection 9 companies have reviewed the varieties. Among them, it is worth noting that although each product has formed a competition pattern of at least three companies, including the original research product, there are still multiple varieties of which only one company has reviewed one of the specifications. A typical example is the injection-use grenadier. Lowe, although the two companies Yangzijiang and Hainan Puli Pharmaceutical passed the consistency evaluation of the variety, the specifications of the two companies were different, Yangzijiang’s 0.25g, and Hainan Puli Pharmaceutical’s 0.5g.
The fifth batch of centralized procurement injection passed/deemed passed consistency evaluation details
Leading companies gather together, with more than 10 varieties of companies listed
From a corporate point of view, the fifth batch of companies that have adopted the most are China Biopharmaceuticals, including Chia Tai Tianqing, Nanjing Chia Tai Tianqing, Chia Tai Fenghai and Chia Tai Qingjiang Enterprise's multiple varieties, and a total of 12 varieties are listed. , Including 7 injections; followed by Yangzijiang Pharmaceuticals, with 11 varieties entering the fifth batch of centralized procurement, with 10 injections; Qilu and Kelun Pharmaceuticals each have 10 varieties with 7 injections entering; Hengrui Pharmaceuticals also has 8 There are 7 injections for each variety. In addition, Kelun Pharmaceutical’s Moxifloxacin Eye Drops and Ceftazidime Injection; Hengrui Pharmaceuticals also reviewed iodixanol, oxaliplatin and other varieties as recently reviewed varieties, just in time for the fifth batch of collection buses , It’s really early to overrate as well as overrate.

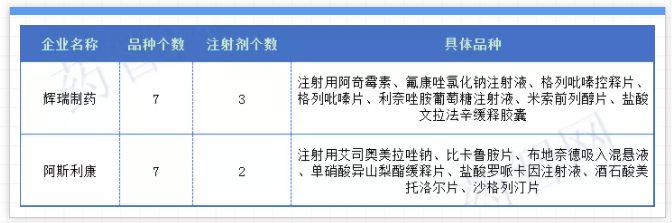
In addition, as far as the original research companies are concerned, the original research companies involved in the fifth batch of centralized procurement include AstraZeneca, Pfizer, GSK, Sanofi, Novartis, Bayer, Boehringer Ingelheim and other more than ten foreign companies; among them, the most noteworthy ones are Pfizer Pharmaceuticals and AstraZeneca each have 7 varieties as the fifth batch of collectively-sourced varieties this time, and are the two pharmaceutical companies with the largest number of collectively-sourced varieties included by foreign companies.
The fifth batch of centralized procurement is the focus of attention regardless of the number of varieties, pharmaceutical formulations and injections, or the competitive landscape of enterprises. Some analysts in the industry pointed out that the fifth batch of centralized procurement plans will be officially released at the end of May or early June, and the bid opening time will be determined; and according to the news of the previous closed-door meeting of the Medical Insurance Bureau, the fifth batch of centralized procurement will be completed before July 1. There is not much time left for the enterprises to prepare for the fifth batch of centralized procurement, and another price war is about to start. It remains to be seen who will be the biggest winner in the end!
Attachment: The latest competitive landscape of the fifth batch of centralized procurement
Note: The deadline for the above data is May 11, if there is any omission, please leave a message for support! Editor in charge: Sanqi









