Qilu, Hengrui, CSPC... 83 varieties have been reviewed! 16 varieties were the first to be reviewed, and a variety of blockbuster injections entered the centralized procurement
June 04, 2021
According to the consistency evaluation analysis system of Yaozhi Data Enterprise Edition, 74 new consistency evaluation acceptance numbers were added in May 2021; 157 approvals (including 38 deemed approved approvals) were over-evaluated. (Attached to the end of the article is the detailed form of consistency evaluation of the May declaration and over-evaluation)
Figure 1 Trend of filing/approval from February 2020 to May 2021
Review details
In May, a total of 157 acceptance numbers passed/deemedly passed the consistency evaluation, involving 83 varieties of 93 companies, of which 16 varieties were the first to pass the evaluation. In terms of over-assessment companies, Qilu Pharmaceutical and Chengdu Better were tied for the first place in May, and all 5 products passed the consistency evaluation. Followed by Chia Tai Tianqing, Jiangsu Hengrui, and CSPC all have 4 products reviewed. Chengdu Better Pharmaceutical Co., Ltd. is a high-tech enterprise specializing in the research and development, production and sales of high-end generic drugs, innovative drugs, Chinese patent medicines and APIs. After years of innovation and development, it has gradually developed into an innovative pharmaceutical company with advanced R&D concepts, strong R&D strength, complete product pipelines, excellent production quality and a sound marketing network. The company has 304 approval documents in China's listed drug database, involving 180 varieties. The company has currently declared/deemedly declared 63 varieties, and 28 varieties including propofol tenofovir fumarate tablets, levetiracetam injection concentrated solution, and ampicillin sodium for injection have been evaluated (including video Same through). Qilu Pharmaceutical has ten major production bases, including preparations, chemical synthesis, biotechnology, antibiotic fermentation, and modern production workshops. Several products such as antibiotics APIs, cephalosporins APIs, and anti-tumor APIs have been domestically and even world-leading Production capacity and production level. The company has 386 approval documents in China's listed drug database, involving 232 varieties. The company has currently declared/deemedly declared 96 varieties, and 58 varieties including Tenofovir disoproxil fumarate tablets, ceftriaxone sodium for injection, and bivalrudine for injection have been evaluated (including video). Same through). The company has ranked first for two consecutive months
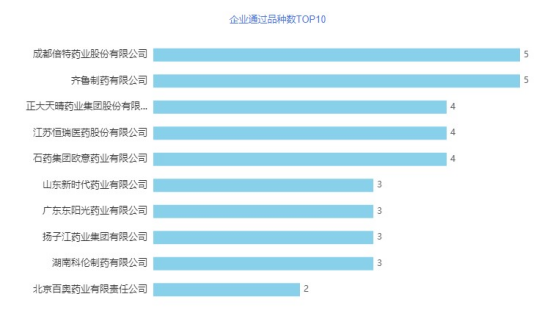
Figure 2 The number of TOP10 products approved by enterprises in May 2021
In terms of varieties, the most intensely competitive variety in May was rivaroxaban tablets, and 7 companies rushed to comment. In addition, 4 companies have reviewed the 4 varieties of ceftazidime for injection. Up to now, 40 companies have applied for consistency evaluation of rivaroxaban tablets, and 23 companies have passed the evaluation. Rivaroxaban tablets, a new type of anticoagulant, used in adult patients with elective hip or knee replacement surgery to prevent venous thrombosis (VTE); used to treat adult deep vein thrombosis (DVT) and pulmonary embolism (PE) ; Used to reduce the risk of DVT and/or PE recurrence in patients with persistent risk of recurrence of DVT and/or PE after completing at least 6 months of initial treatment; used in non-valvular rooms with one or more risk factors In adult patients, to reduce the risk of stroke and systemic embolism; under well-controlled conditions with warfarin, compared with warfarin, there are limited data on the relative effectiveness of rivaroxaban in reducing the risk of stroke and systemic embolism. This variety has ranked first for two consecutive months.
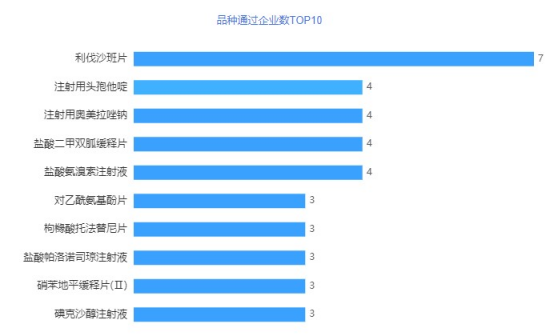
Figure 3 TOP 10 companies passing the variety of products in May 2021
Application for 50 varieties, nearly 60% of injections
In May 2021, CDE added 74 acceptance numbers for consistency evaluation, involving 50 varieties of 47 companies, of which injections accounted for the number one.
Figure 4 Details of dosage forms declared in May 2021
In terms of the declared varieties, in May 2021, 8 acceptance numbers of cefuroxime sodium for injection were accepted. In addition, ceftriaxone sodium for injection, omeprazole sodium for injection, and lidocaine hydrochloride injection were all accepted. There are 3 acceptance numbers that have been undertaken. For details, see the figure below.
Figure 5 TOP10 number of variety declaration acceptance numbers in May 2021
Cefuroxime sodium for injection is suitable for unidentified bacterial infections or infections caused by sensitive bacteria. In addition, this product can also be used to prevent infections after various operations. Under normal circumstances, this product can be effective when used alone, but if the condition is suitable, it can be combined with aminoglycoside antibiotics, or combined with metronidazole (oral, suppository, and injection), especially for colon surgery to prevent infection. There are currently 314 approvals for cefuroxime sodium for injection in China, involving 77 companies. At present, 7 companies have applied for consistency evaluation, and Zhejiang Huidisen Pharmaceutical Co., Ltd. and Shandong Runze Pharmaceutical Co., Ltd. have passed the evaluation. From an enterprise perspective, in May, Qilu Pharmaceutical Co., Ltd. was accepted for the consistency evaluation of 5 acceptance numbers, again ranking first on the list. Hainan Hailing Chemical Pharmaceutical Co., Ltd. has 4 acceptance numbers that have been accepted for consistency evaluation, ranking second. Hailing Pharmaceutical mainly focuses on the production and sales of high-quality prescription antibiotic drugs. There are more than 130 products in total, including antibiotics, cardiovascular, gastrointestinal and topical creams and other types of products. Hailing has established a comprehensive and huge sales and distribution network in China. Hailing sells its products to 31 provinces, autonomous regions and municipalities in China through more than 600 distributors. The enterprise has currently declared/deemedly declared 7 varieties, and 5 varieties have been evaluated (including deemed passed). In addition, Jinhong Pharmaceutical Co., Ltd. has 3 acceptance numbers accepted. Jinhong Pharmaceutical Co., Ltd. is a modern pharmaceutical enterprise integrating drug research and development, production and sales. At present, the company has 55 approval documents in China's listed drug database, involving 25 varieties. 7 varieties have been declared/deemedly declared, of which cefixime dispersible tablets and cefixime granules have been evaluated (including deemed passed).
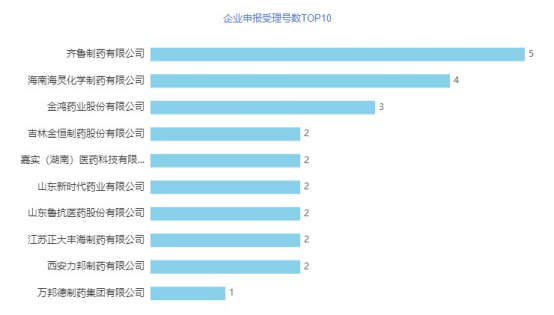
Figure 6 TOP10 number of enterprise application acceptance numbers in May 2021
Attached Table 1: Details of the consistency evaluation passed (including deemed passed) in May 2021
Attached Table 2: Details of the consistency evaluation of the declaration in May 2021
Data as of June 1, 2021
Data source: Yaozhi Data Generic Drug Consistency Evaluation and Analysis System










