Baike Biologics purchase day: there is no need to use the "nasal spray new crown vaccine", the shingles vaccine is the core growth point
June 16, 2021
A few days ago, at a press conference held by the Joint Prevention and Control Mechanism of the State Council, a reporter had just asked about the latest research status and characteristics of my country's atomized inhaled COVID-19 vaccine. The CDC researcher Shao Yiming responded that on the day of the purchase of Baike Biology, he declared that it was a "nasal spray new crown vaccine." As everyone knows, the core growth force of Beike Biology is not here at all. Recently, the new crown vaccine that does not require injections has become popular. The CDC researcher Shao Yiming also conducted a wave of official science on this: atomized inhalation or nasal spray vaccine is one of the five new crown vaccine research technical routes laid out in our country in the early stage. Relevant research and development units are developing new vaccines with nasal spray or atomization as planned. Phase I and II clinical trials are already in progress. Research data is collected for statistical analysis, and expert demonstrations are organized in a timely manner to evaluate the safety and immunity of this administration method. Originality, and then in the third phase of clinical trials or emergency use process, to further verify its effectiveness.

Compared with the intramuscular injection dosage form, it is only an improvement in the dosage form, and the basic formulation has not changed, only the way of administration is to use aerosol inhalation instead of intramuscular injection. Among them, the nasal spray attenuated influenza virus carrier new crown vaccine developed by Xiamen University, Hong Kong University and Beijing Wantai Company has also completed phase one and two clinical trials. Currently, it is applying for a Phase III clinical trial overseas to further verify the effectiveness of this vaccine. Chen Wei, an academician of the Chinese Academy of Engineering, said that compared to the humoral and cellular immunity formed by the injectable new crown vaccine, the inhaled new crown vaccine can also form mucosal immunity. This triple immunity is the most ideal state. In addition, there is less demand for external conditions and higher accessibility.
The income of varicella vaccine has an absolute advantage, the other varieties are weak
On June 15th, Baike Biotechnology opened a subscription with an issue price of 36.35 yuan. In fact, as early as last year, the market had news that Changchun Hi-tech planned to spin off its vaccine-producing subsidiary Baike Biotechnology. Last month, it was mentioned in the announcement that its subsidiary, Beike Biotechnology, intends to obtain the new coronavirus vaccine (PIV-5 vector) and related technologies for the vaccine (PIV-5 vector) developed against the new coronavirus mutant strain with Guangzhou Sianxin. Sign the "Licensing Cooperation Agreement" with the exclusive license right in the license area. According to the announcement, related technologies can be administered through the nasal mucosa to induce humoral immunity, cellular immunity, and mucosal immunity. A review of the performance of the last three years found that Baike Biotechnology achieved revenue of 976 million yuan in 2019, a decrease of 4.24% year-on-year. The main reason was that the sales amount of rabies vaccine decreased from 152 million yuan in 2018 to 27 million yuan in 2019. Revenues will resume growth in 2020, achieving revenue of 1.441 billion yuan that year, a year-on-year increase of 47.73%. The study found that the flu vaccine was listed that year and contributed about 333 million yuan in revenue to be the main reason for the company's revenue growth.

This listing plan to raise funds of 1.681 billion yuan, mainly used to expand varicella vaccine, zoster live attenuated vaccine, adsorption acellular DPT (three-component) combined vaccine, influenza vaccine (liquid preparation), rabies vaccine and Hib vaccine production capacity and product development under research. However, there is one point worth noting in the financial data such as revenue. From 2018 to 2020, the proportion of 100 grams of biological varicella vaccine in the main business income has always exceeded 75%. At the same time, the company's varicella vaccine is also facing increasingly fierce competition. As of the signing date of the prospectus, five manufacturers in the domestic varicella vaccine market have been approved for listing, including Beike Bio, Shanghai Institute, Changchun Qijian, Shanghai Rongsheng, and Dalian Kexing. According to the Dongguan Securities Research Report, there are five other domestic companies that are conducting research and development on varicella vaccine projects. In 2020, the percentage of batches issued for the 100 grams of biological varicella vaccine will account for 32.01%, temporarily ranking first in the industry.
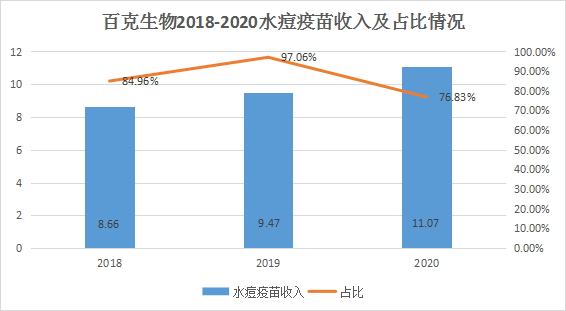
However, the competitiveness of other vaccines on sale is relatively weak, and most of the 12 vaccines under development and 2 fully human monoclonal antibodies for the prevention and control of infectious diseases are also in the preclinical research stage. In response, the board of Baike Biologics replied that China's varicella vaccine market still has room for growth. At present, the company's nasal spray flu vaccine has been on the market in the second half of 2020, accounting for 23.10% of the current main business income. In addition, the company has carried out a number of upgrades and optimizations to the rabies vaccine, which has been submitted to the National Drug Evaluation Agency (CDE). It is expected to resume production in the second half of 2021 and resume sales in the first half of 2022.
Compared with the nasal spray new crown vaccine, the shingles vaccine is the growth point
Perhaps because of the above, the sales cost of 100 grams of biological products has remained high throughout the year. From 2018 to 2020, they were 517 million yuan, 449 million yuan, and 578 million yuan, accounting for 50.71%, 46.07%, and 40.09% of operating income, respectively. Compared with competing companies in the same period, it is higher, reflecting to a greater extent that the company's products are facing fierce competition. In addition, as a veteran vaccine company, after the Changsheng Biological incident, Changchun Qijian and Shanghai Stock Exchange increased their market share significantly. Beijing Kexing is also a newcomer, but Baike Biology is more than stable and not aggressive enough. And instead of hoping that only obtaining the authorized "nasal spray new crown vaccine" will have an impact on the company's core competitiveness, perhaps the shingles vaccine is more promising. There are currently two vaccines for preventing shingles on the global market, namely Merck’s Zostavax and GlaxoSmithKline’s Shingrix. It was approved by the FDA in 2006 and 2017, and the latter has an efficiency rate of 90%, making it a blockbuster product. In China, there is still a lack of effective preventive measures for the disease. The recombinant shingles vaccine Shingrix, which was approved for marketing in 2019, has become the first domestic shingles vaccine to be marketed, and will be released in July 2020. Completed the domestic market vaccination for the first time in May. However, due to various factors such as price and quantity, the patient's demand for domestic vaccines is becoming more and more urgent.
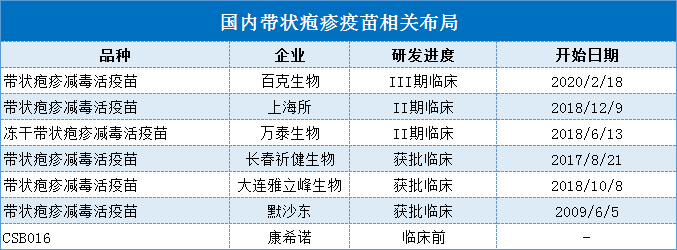
At present, the domestic shingles vaccines under research are all live attenuated vaccines. The research and development progress of Beike Biological, Shanghai Institute and Wantai Biological is relatively advanced, and the research and development of Baike Biological Live attenuated zoster vaccine has entered clinical practice. In Phase III, the progress is relatively advanced. If the research and development is successful, it may be the real growth point of Beike Biology.
Editor in charge | Penicillin
Source: Yaozhi.com






